
Carbamazepine Hypersensitivity RealFast® Assay
These assays (HLA-A*31:01 RealFast Assay™ and HLA-B1502 RealFast™ Assay) are for the identification of genes important in the adverse reaction to Carbamazepine. Carbamazepine is an anticonvulsant drug, which is generally prescribed for the treatment of bipolar disorder, epilepsy, and trigeminal neuralgia. There are a number of geographical populations particularly at risk for adverse reactions from Carbamazepine:
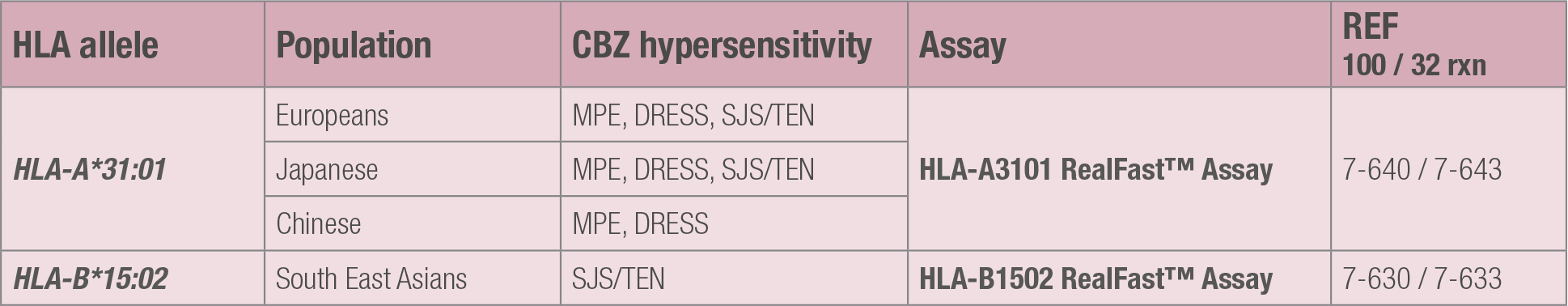
-The human leukocyte antigen allele HLA-A*31:01 is associated with Induced Maculopapular Exanthema, Drug Reaction with Eosinophilia and Systemic Symptoms, and Toxic Epidermal Necrolysis in Japanese and European populations.
-The human leukocyte antigen allele HLA-A*31:01 is associated with Maculopapular Exanthema and Drug Reaction with Eosinophilia and Systemic Symptoms in Chinese populations.
-The human leukocyte antigen allele HLA-B1502 is associated with Drug Reaction with Eosinophilia and Systemic Symptoms, and Toxic Epidermal Necrolysis in many Asian populations.
Carbamazepine Hypersensitivity RealFast® Assay Brochure
- Kits are based on reverse-hybridization of biotinylated PCR products.
- Minimal equipment required (thermal cycler, shaking water bath).
- Technology combines probes for mutations and controls in a parallel array of allele-specific oligonucleotides.
- Functions using immobilized oligos on a teststrip.
- Strips show mutations by enzymatic color reaction visible to the naked eye.
- Proprietary software package (Evaluator™) that aids in data interpretation, storage, and archiving.
Publications
HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans (McCormack, 2011)
Allelic Variants Detected
HLA-A*31:01
HLA-B*15:02